Top: Some of the logs LiberTrace red-flagged for having multiple issues but the FDA still allowed to be shipped. The DayLight/Derick Snyder
By Esau J. Farr
MONROVIA – The Forestry Development Authority (FDA) permitted a company to export round logs mid-last year. However, the regulator ignored its computerized system—known as LiberTrace—red-flagged over 60 percent of the timber.
Out of the total 431 logs, Iroko Timber and Logging Corporation submitted for two shipments, LiberTrace identified 267 as problematic.
LiberTrace, which tracks logs from their sources to final destinations, found the logs’ details were inconsistent with the system’s information. Most of the logs had not been recorded during a pre-export inspection.
For instance, some logs had their butt-end diameters different from what Iroko declared. Others had volumes different from the ones submitted, while other logs had discrepancies with the lengths the Nigerian-owned company declared.
But the LiberTrace analysis and the export specs detailing each log shipped establish that the FDA allowed the tainted logs to go.
The combined 431 logs with a 2,549-cubic-meter volume, were loaded at the Port of Greenville, Sinoe County and departed on April 27 and July 2, 2024, on the Panamanian cargo ship MV Nimeh, destined for Bangladesh.
‘Nothing to add’
Based on the FDA’s standard operating procedures (SOPs) the regulator should have investigated the red flags and sought correction. If not, the SOPs provide the export to be disapproved. “Wood products that are not compliant with the legality definition shall not be authorized for export,” according to SOPs for export.
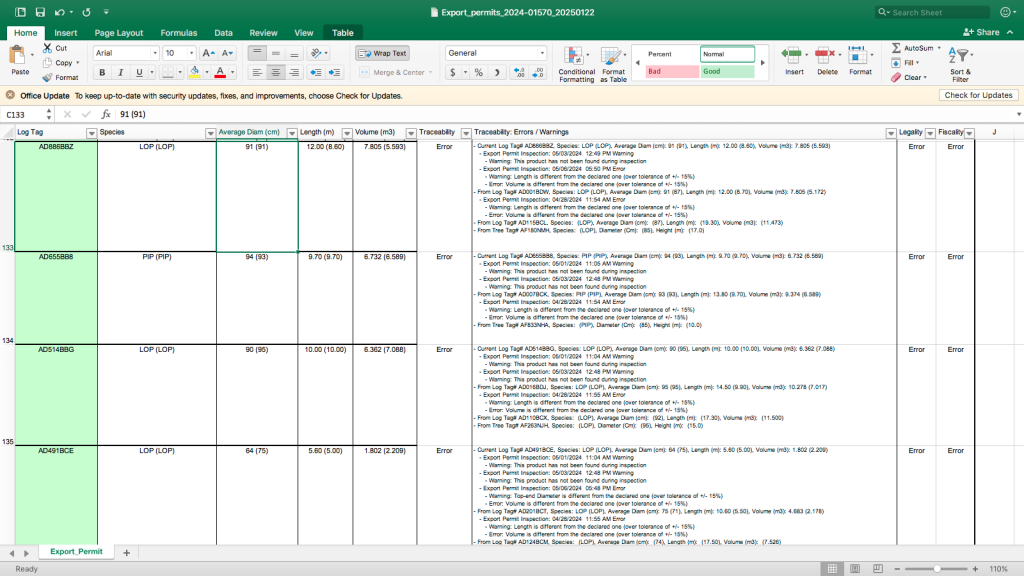
The SOPs allow for the FDA to override LiberTrace’s alarms. However, in such a case, the FDA is required to record the justification for overriding the red flags for auditing. Screenshots of LiberTrace’s history of the logs prove there were no justifications for the FDA’s decision to approve the exports.
Those standards contribute to LiberTrace ensuring tax-complaint companies’ logs are legal, not just traceable. LiberTrace plays a critical role in the forestry sector, particularly in combating illegal logging and enhancing transparency in the timber trade. SGS, a Swiss verification company, built the system and the FDA co-manages it.
Confronted with the red flags, Theodore Nna, SGS’ project manager, did not respond to queries. Nna did the same last year in a similar incident. He had sarcastically offered The DayLight a tutorial in interpreting LiberTrace’s data and analysis.
The FDA Managing Director Rudolph Merab declined to speak on the matter. “I believe my team handled this Iroko issue last year…,” Merab said in a WhatsApp chat. “I have nothing new to add!”
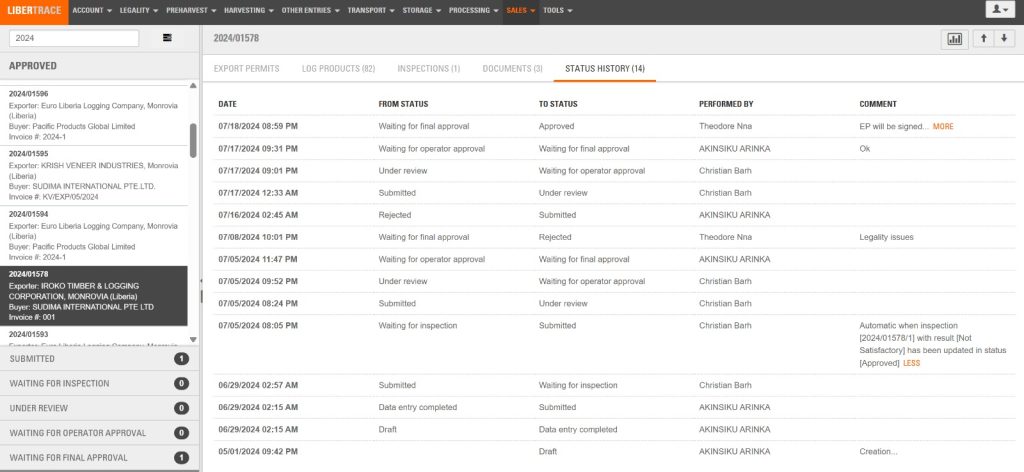
Last year, the FDA dismissed reports as a “misinterpretation” of data. It argued that the errors and warnings LiberTrace sounded were routine “minor occurrences.”
Similarly, Iroko did not return emailed questions. The company had initially responded to the DayLight’s inquiries but ceased after the newspaper exposed a series of its wrongdoings.
This investigation adds to the logs’ taint and Iroko’s notoriety. A previous investigation found the logs spent over a year in the Central River Dugbe Community Forest in Sinoe County’s Jaedae District. One unearthed Iroko owed local people a good sum. Another revealed an Iroko shareholder was unqualified for logging over a co-ownership of a company punished for fraud.
This story was a production of the Community of Forest and Environmental Journalists of Liberia (CoFEJ).





Facebook Comments